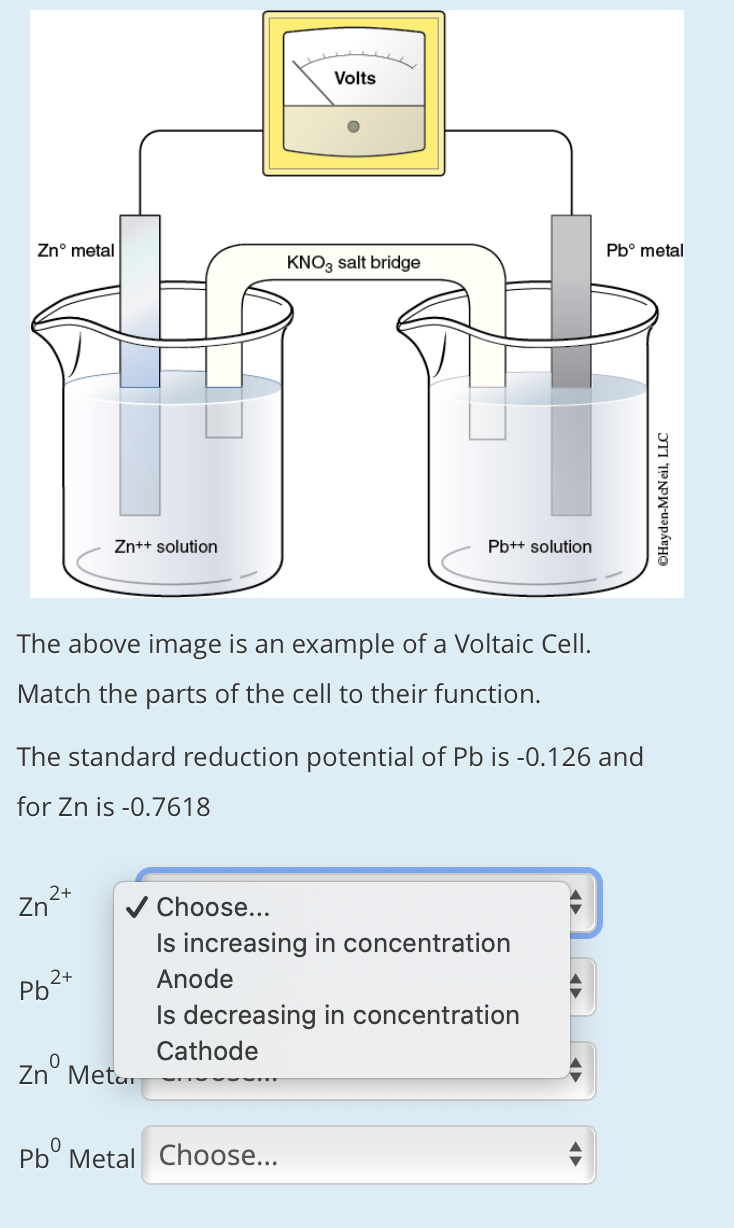
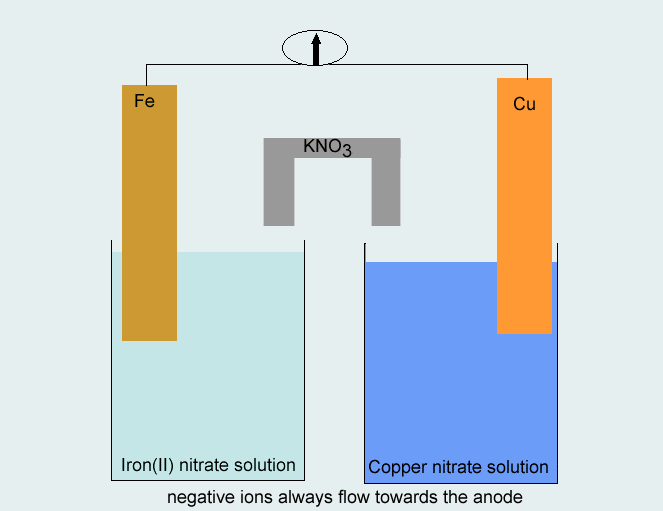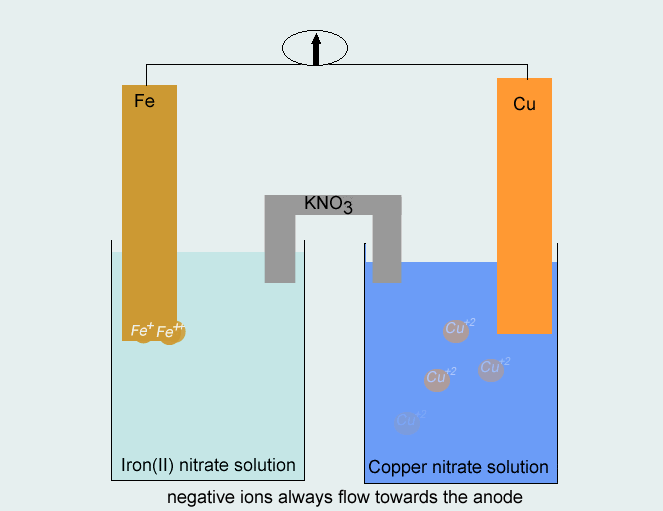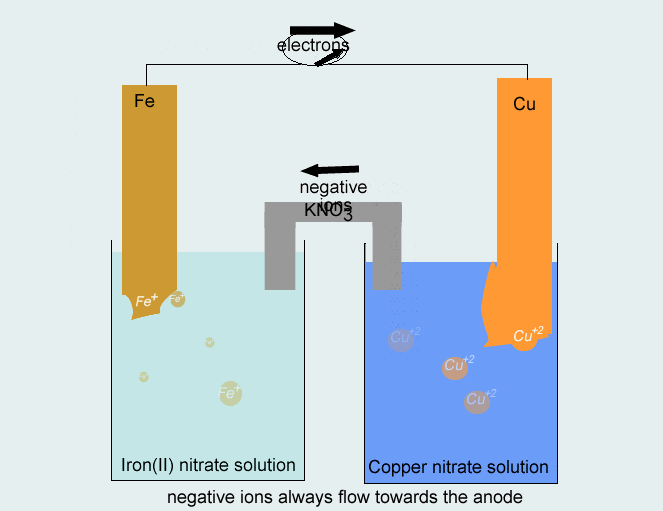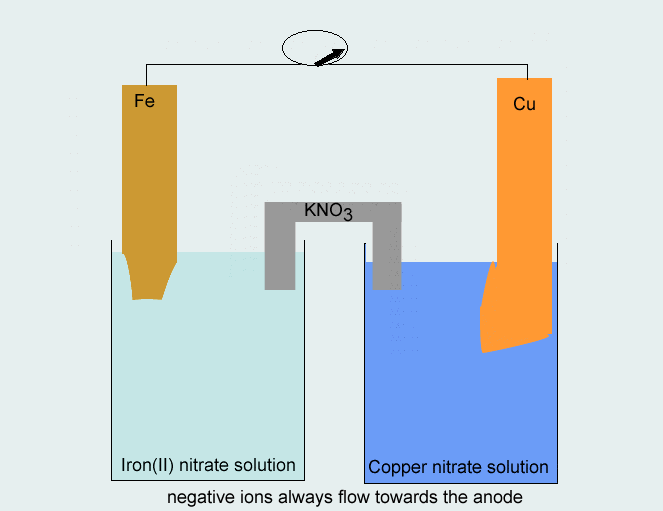
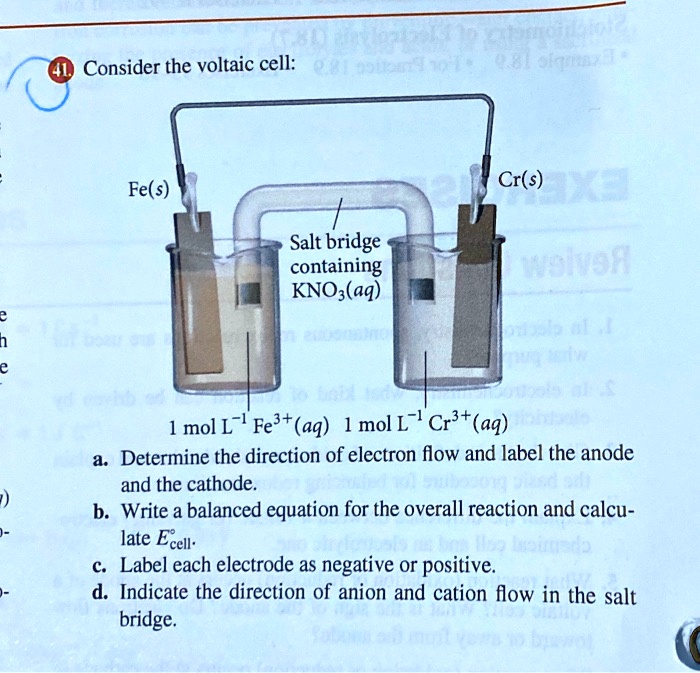
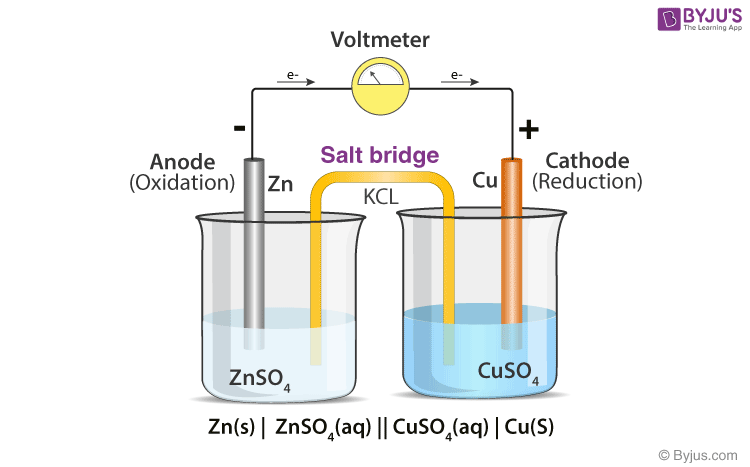
Consider the voltaic cell: 1 M Fe3+ 1 M Cr3+ Fe(s) Cr(s) Salt bridge containing KNO3(aq) | StudySoup

physical chemistry - Why is it important to use a salt bridge in a voltaic cell? Can a wire be used? - Chemistry Stack Exchange
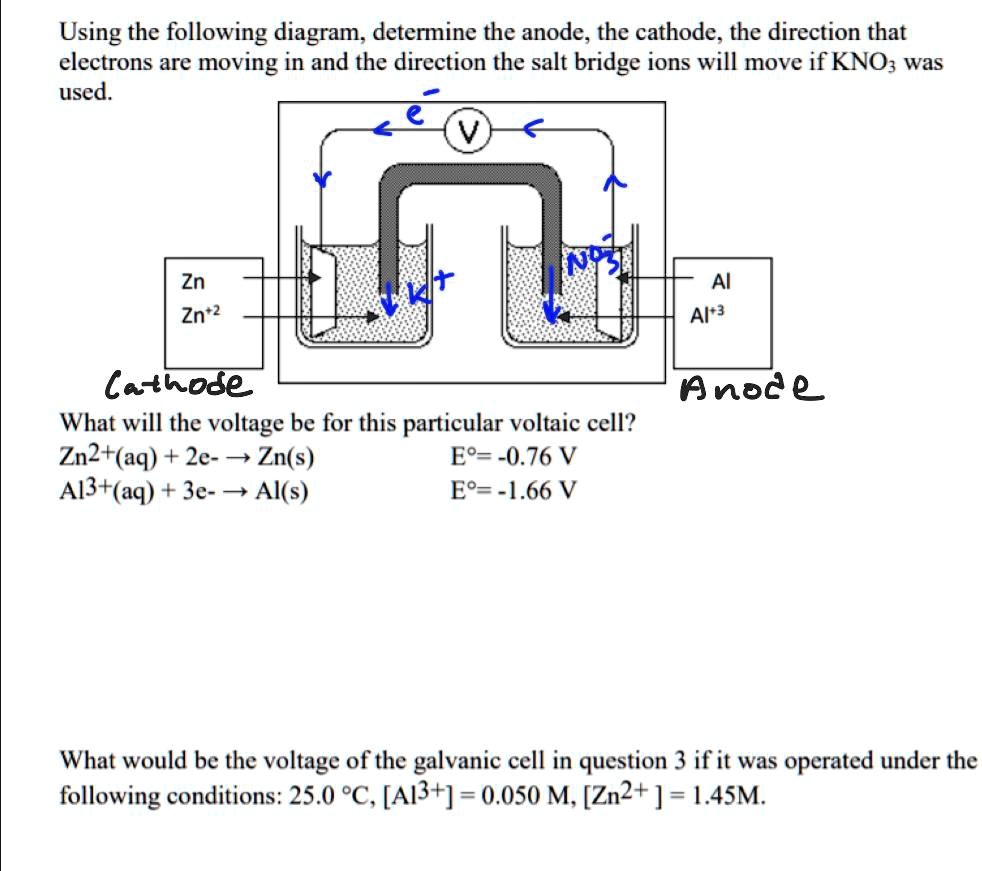
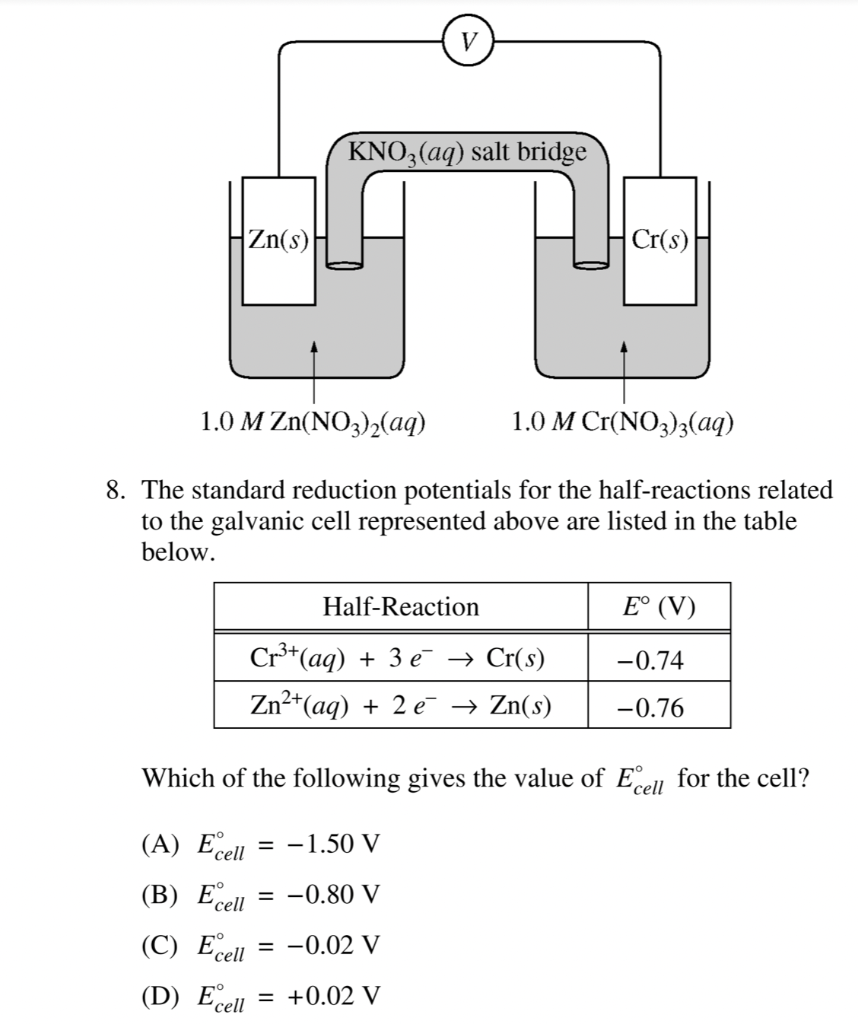
SOLVED: Using = the following diagram, determine the anode, the cathode; the direction that electrons are moving in and the direction the salt bridge ions will move if KNOz was used, Zn
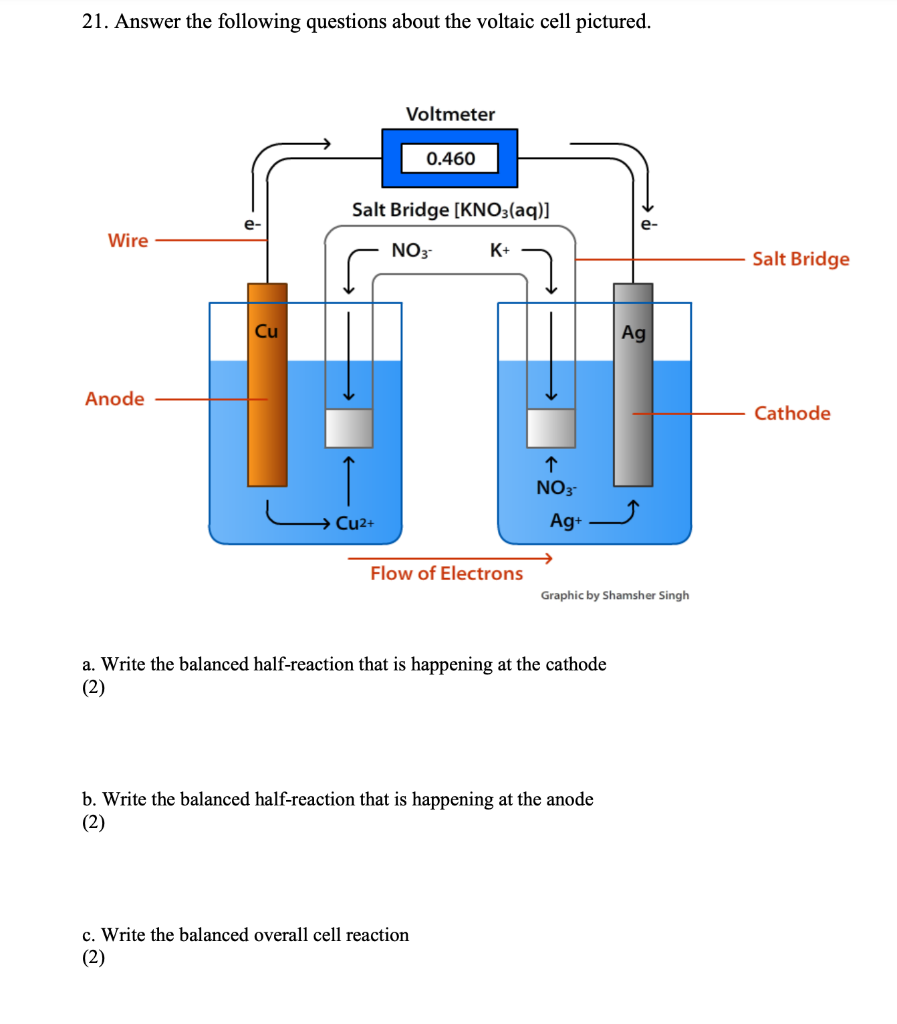




![Solved Voltmeter Wire 0.460 Salt Bridge [KNO3(aq)] NO3 K+ e- | Chegg.com Solved Voltmeter Wire 0.460 Salt Bridge [KNO3(aq)] NO3 K+ e- | Chegg.com](https://media.cheggcdn.com/media/80b/80bf4639-9e4d-4fd2-9391-8ac54bd7d867/php7d36Tc)

![Solved Flow of Electrons Salt Bridge [KNO3(aq)] e- e- Wire | Chegg.com Solved Flow of Electrons Salt Bridge [KNO3(aq)] e- e- Wire | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2F41e%2F41e13ad9-193d-446b-ae98-025eec171dc7%2FphpVmmSrB.png)