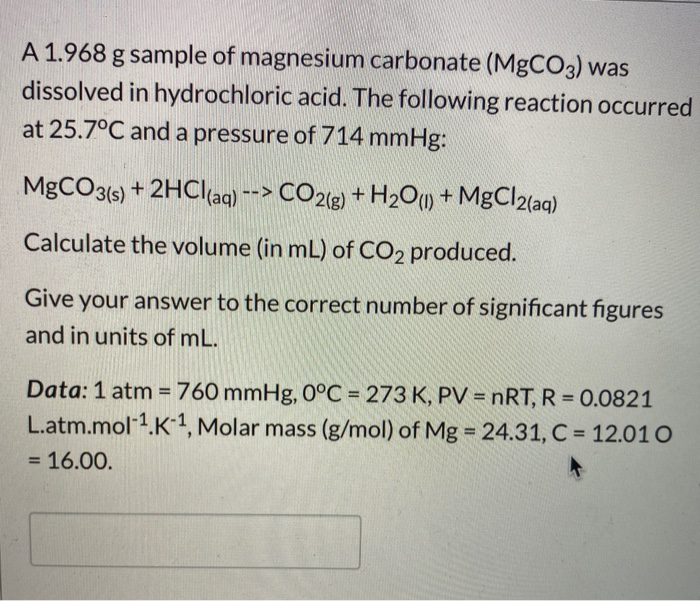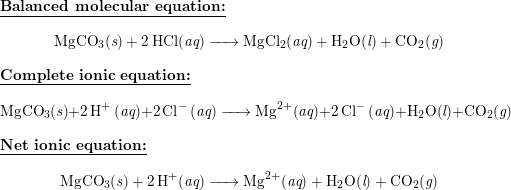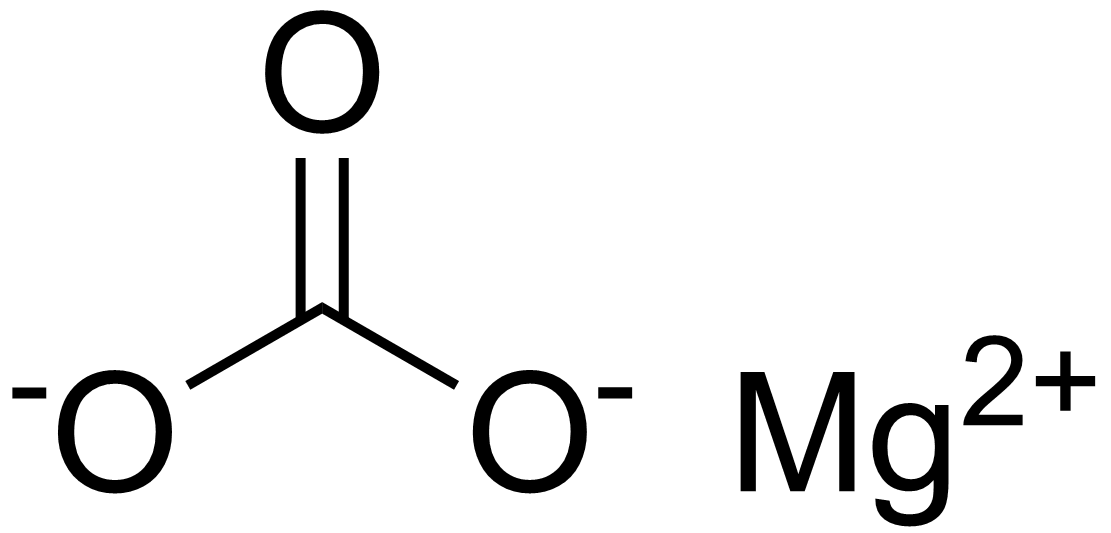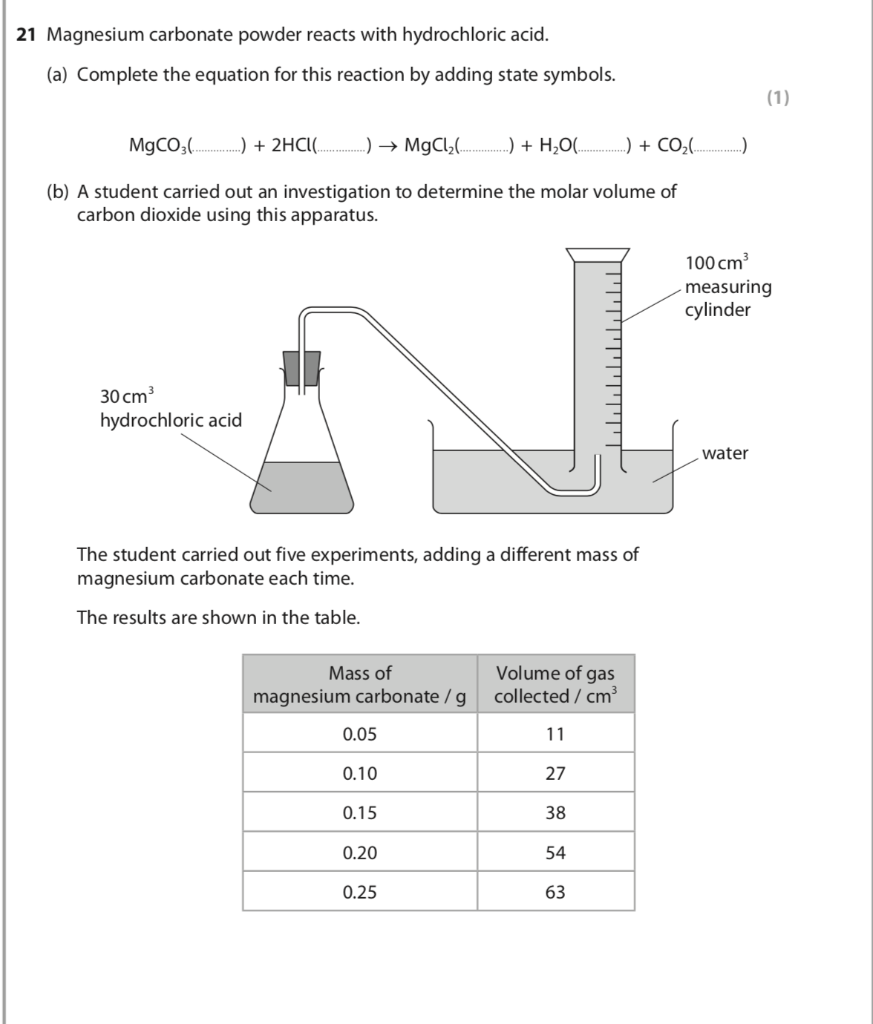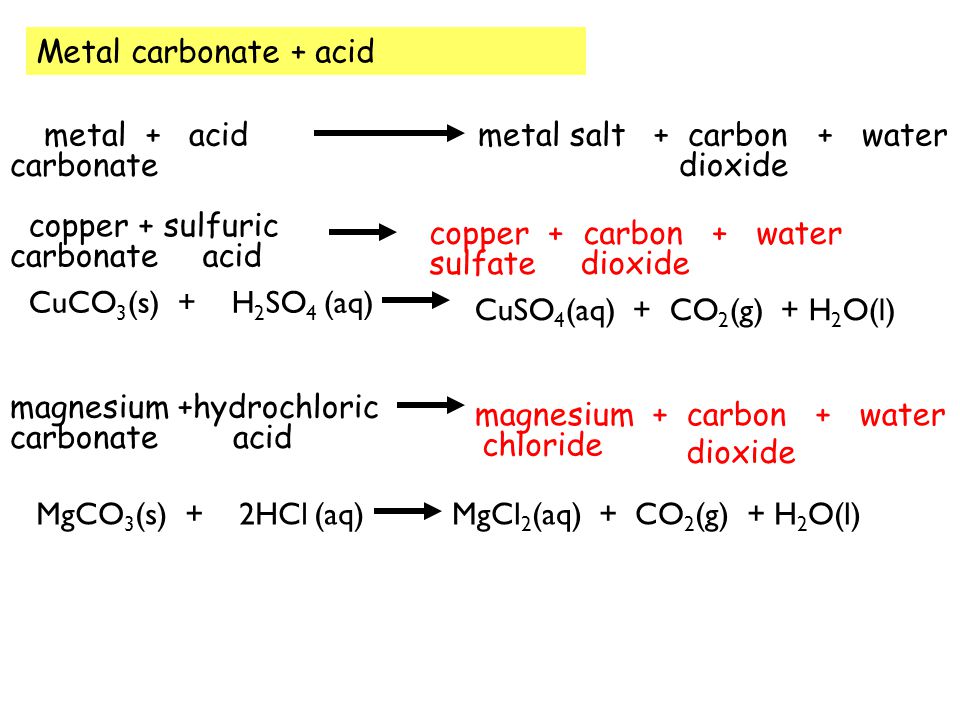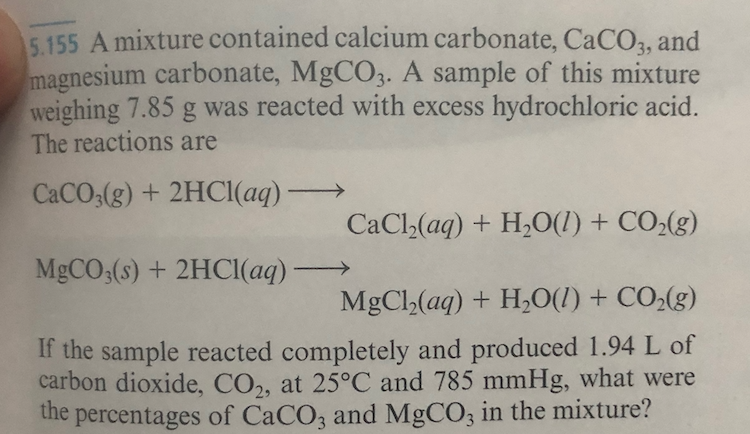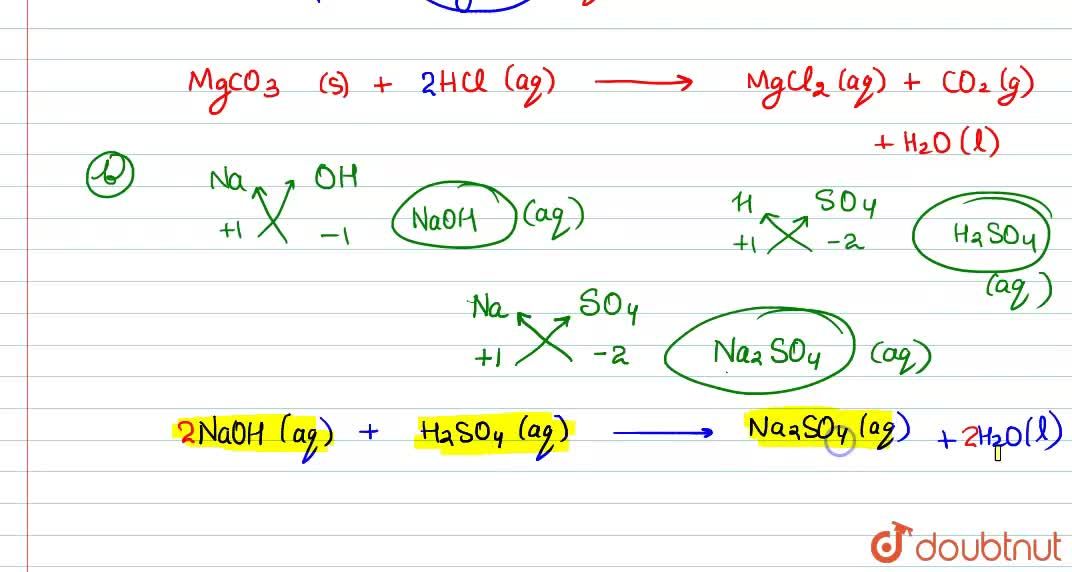
Write the balanced equations for the following reactions, and add the state symbols : (a) Magnesium carbonate reacts with hydrocloric acid to produce magnesium chloride, carbon dioxide and water. (b) Sodium hydroxide
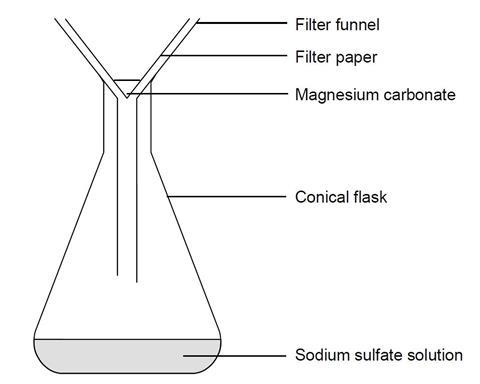
Making magnesium carbonate: the formation of an insoluble salt in water | Experiment | RSC Education

Magnesium carbonate reacts with hydrochloric acid to form magnesium chloride, carbon dioxide and water Translate and balance the equation - Science - Chemical Reactions and Equations - 12554199 | Meritnation.com