Data Integrity and Privacy – compliance with 21 CFR Part 11, SaaS/Cloud Tickets, Wed, Jan 25, 2023 at 1:00 PM | Eventbrite

Reduce costs for compliance with data integrity: 21 CFR Part 11, SaaS/Cloud Tickets, Thu, Nov 17, 2022 at 1:00 PM | Eventbrite

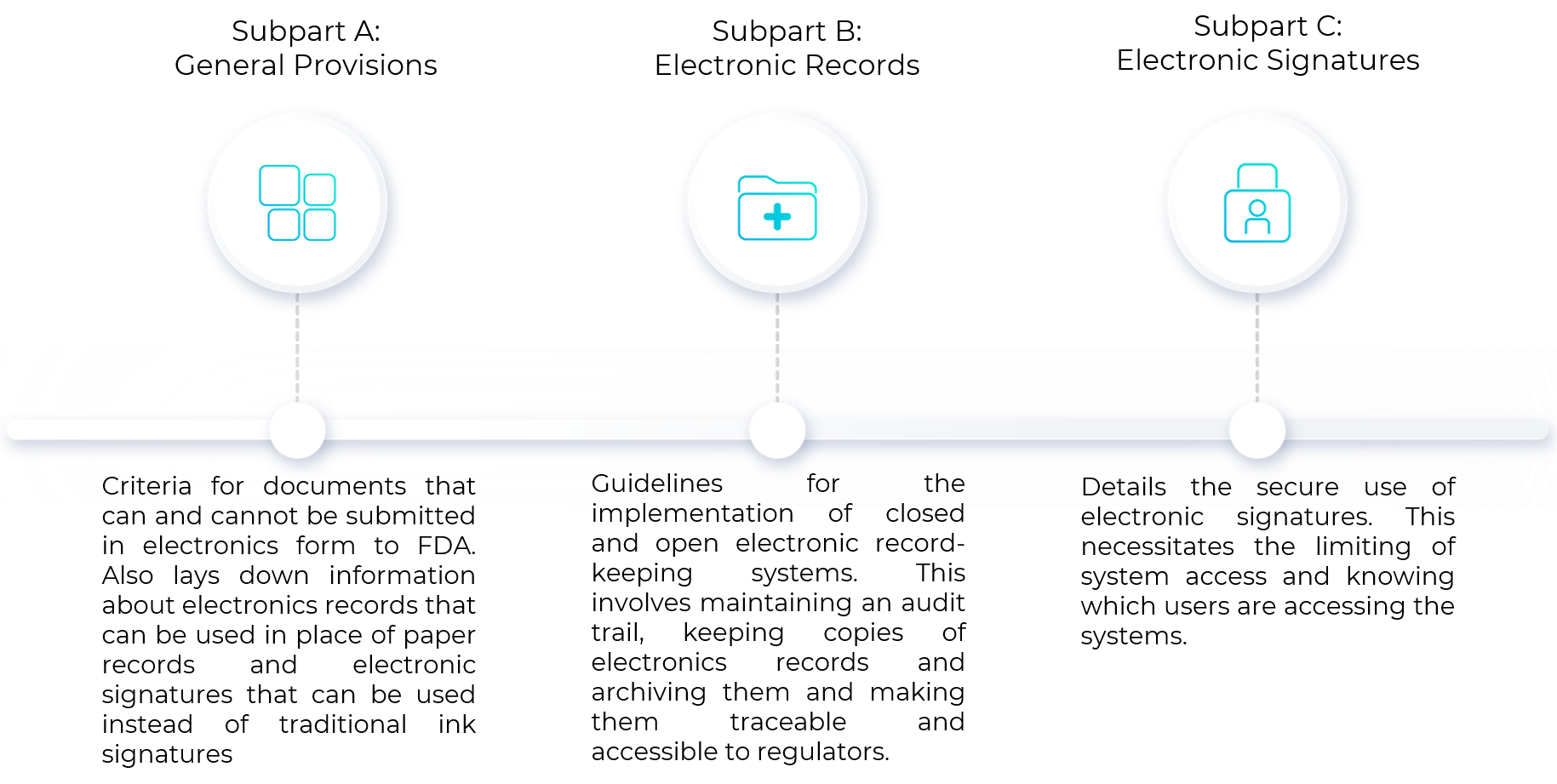
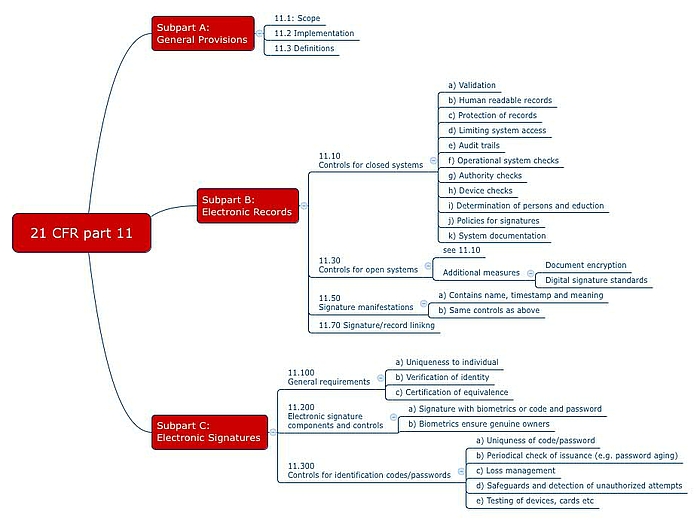
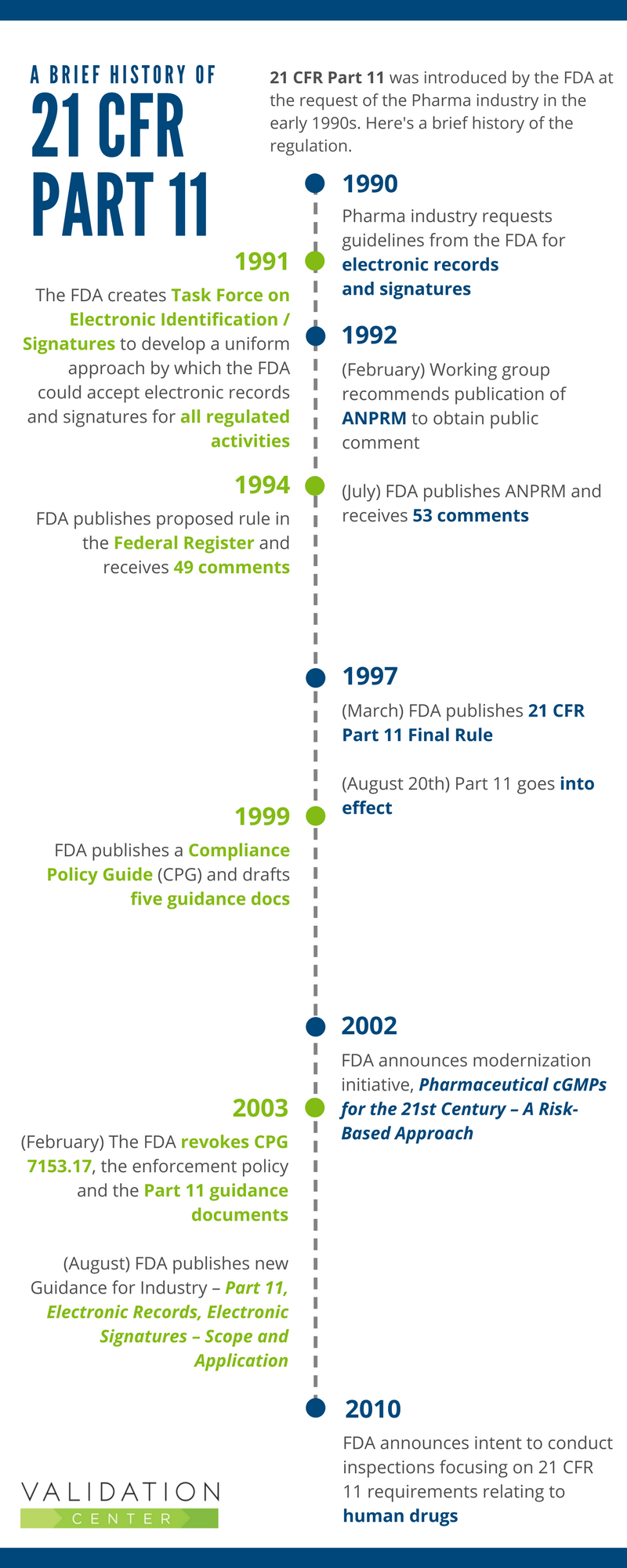
21 CFR Part 11 : Electronic Signature & LMS compliance - DOKEOS - LMS & E-learning Suite for growing companies