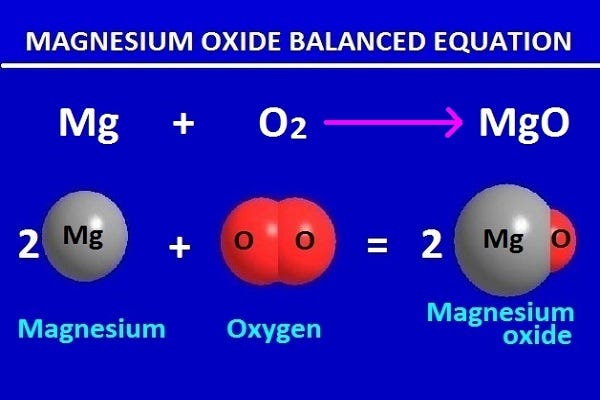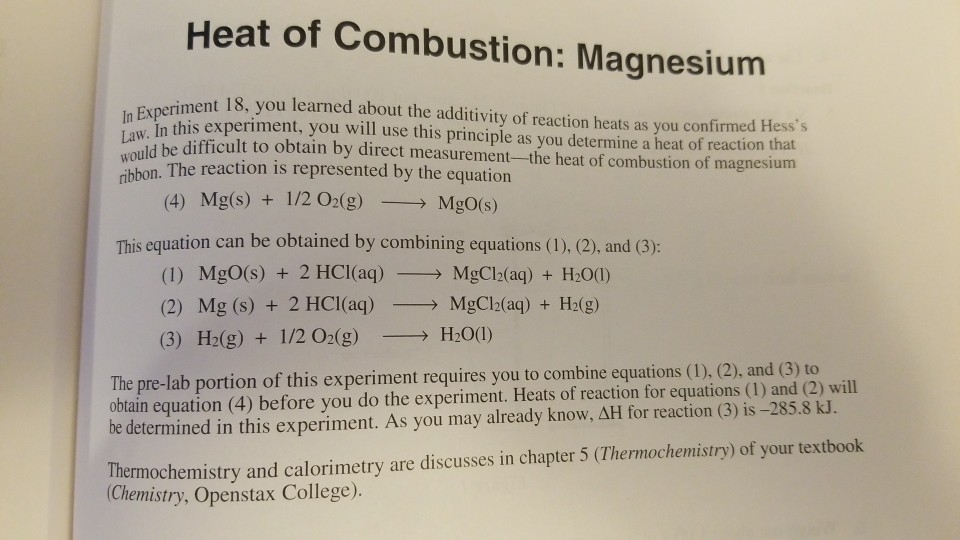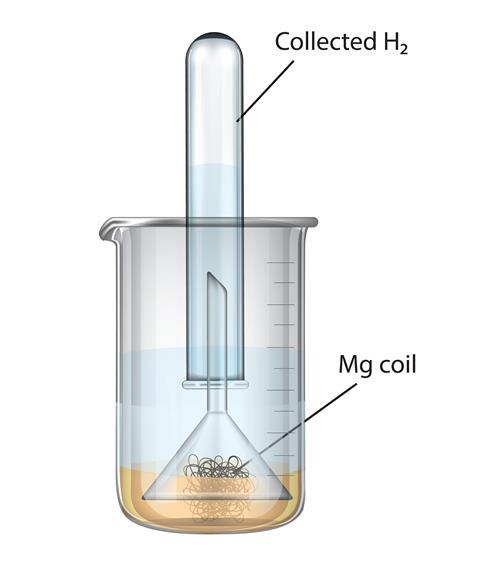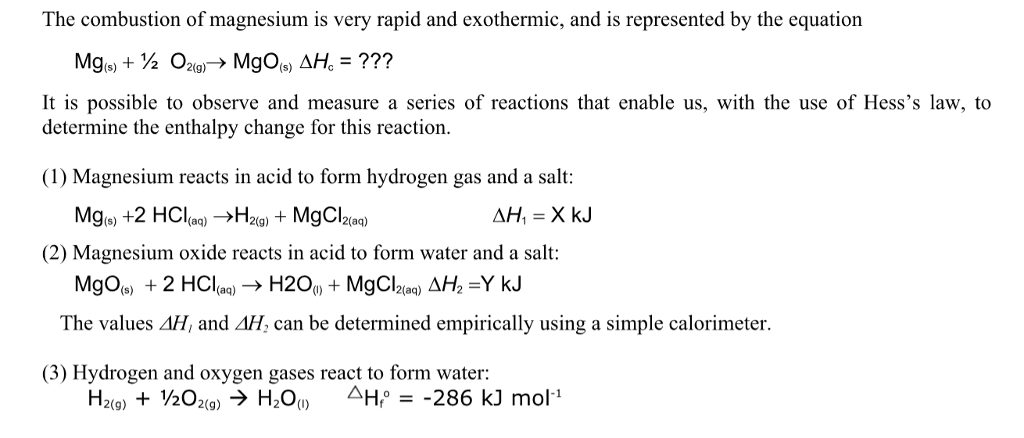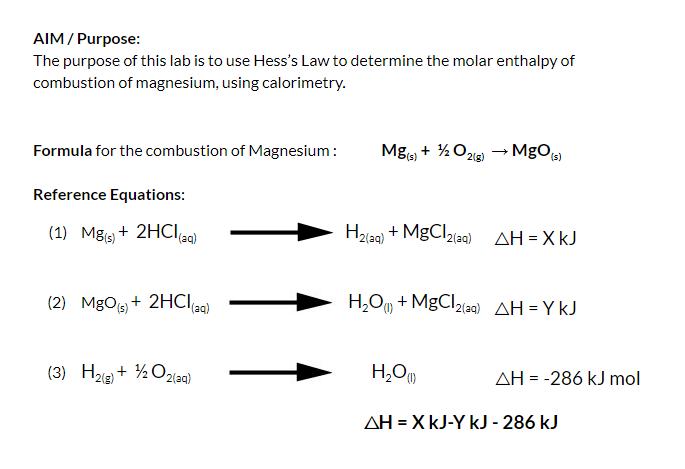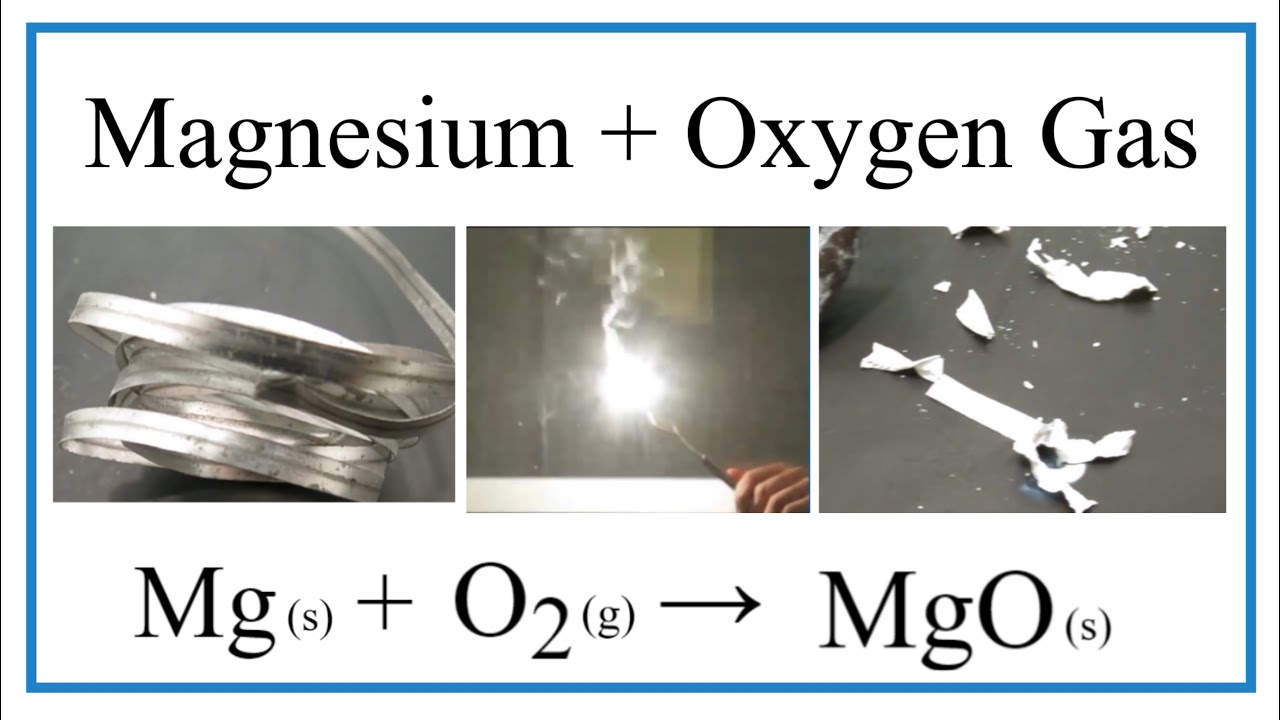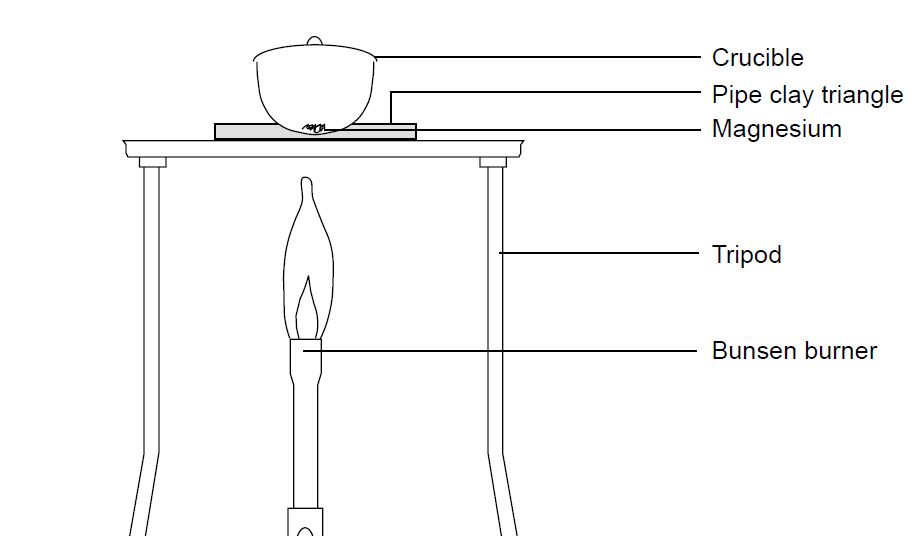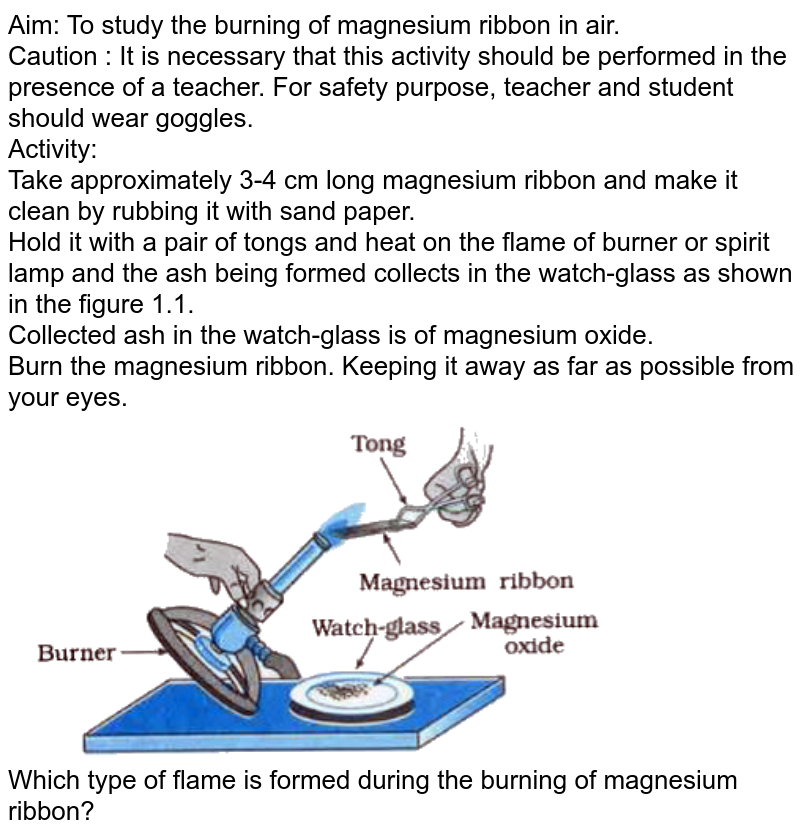
Aim: To study the burning of magnesium ribbon in air. Caution : It is necessary that this activity should be performed in the presence of a teacher. For safety purpose, teacher and
![SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)- SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)-](https://cdn.numerade.com/ask_previews/1bda9caf-ebe2-4ef5-bcb8-b82a12a7fae5_large.jpg)
SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)-

Write a balanced chemical equation for the following chemical reaction : Magnesium burns in oxygen - YouTube
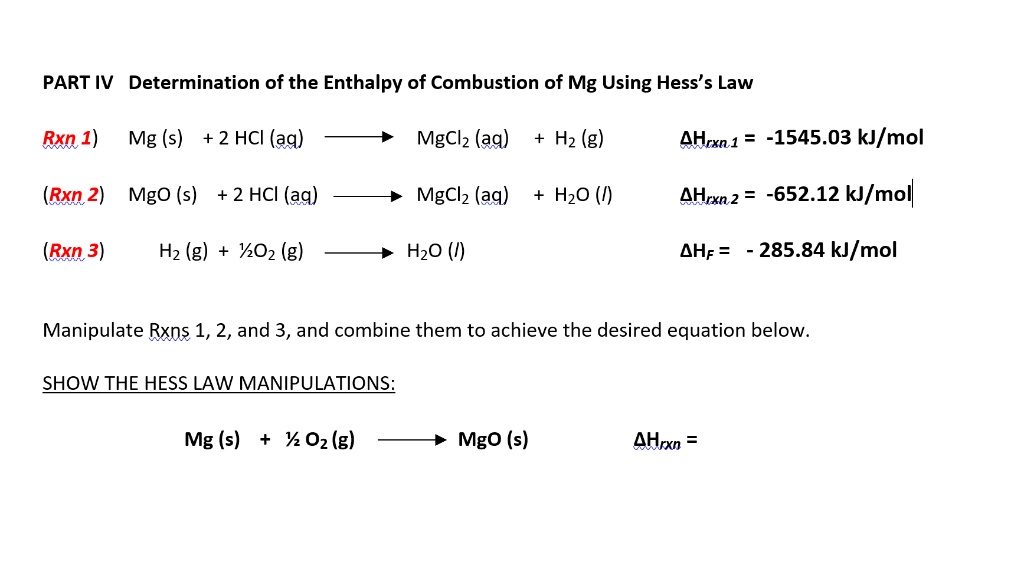
SOLVED: PART IV Determination of the Enthalpy of Combustion of Mg Using Hess's Law Rxn 1) Mg (s) +2 HCI (aq) Mgclz (aq) Hz (g) AHrxn 1 -1545.03 kJ/mol (Rxn 2) Mgo (
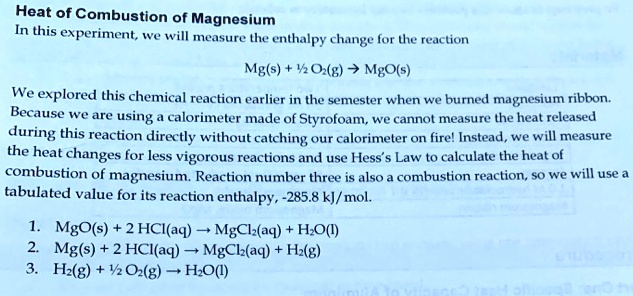
SOLVED: Heat of Combustion of Magnesium In this experiment; we will measure the enthalpy change for the reaction Mg(s) "0 (H) MgO(s) We explored this chemical reaction earlier in the semester when

Burning magnesium in a Bunsen flame and other flame experiments | Chem 13 News Magazine | University of Waterloo

SOLVED:Magnesium burns in air to produce a bright light and is often used in fireworks displays. The combustion of magnesium can be described by the following thermochemical equation: 2 Mg(s)+O2(g) ⟶2 MgO(s)

Skills Lab: Combination of Matter Essential Question: When I burn something, does it get heavier or lighter? Mg + O ppt download