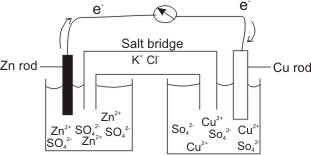
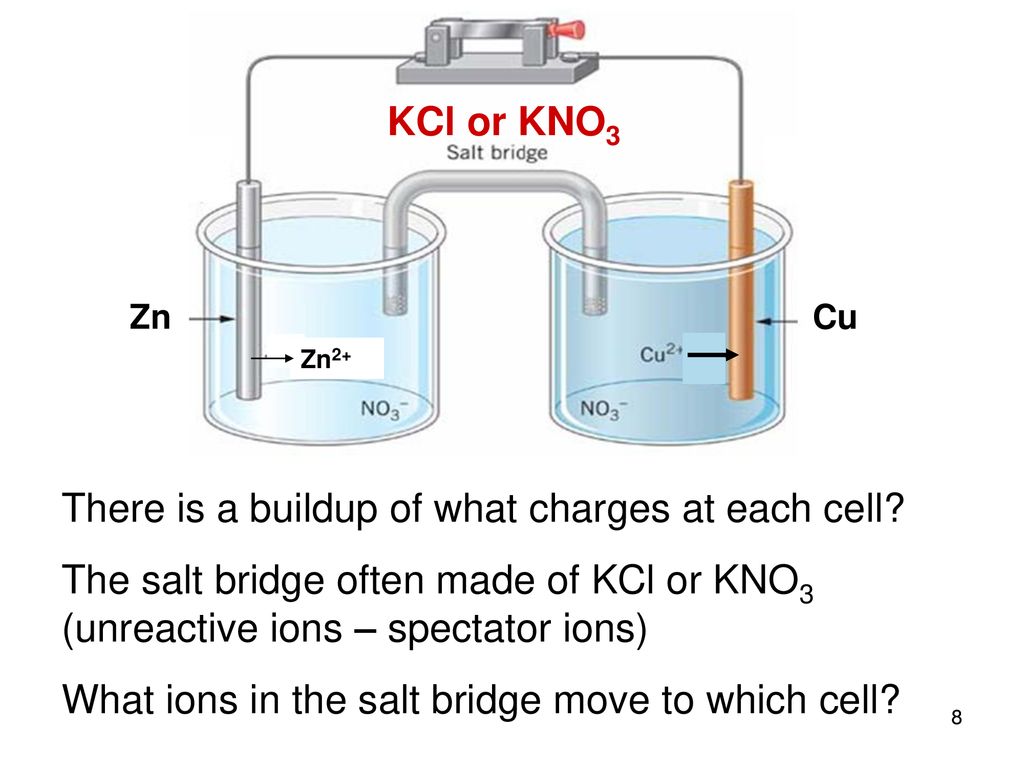
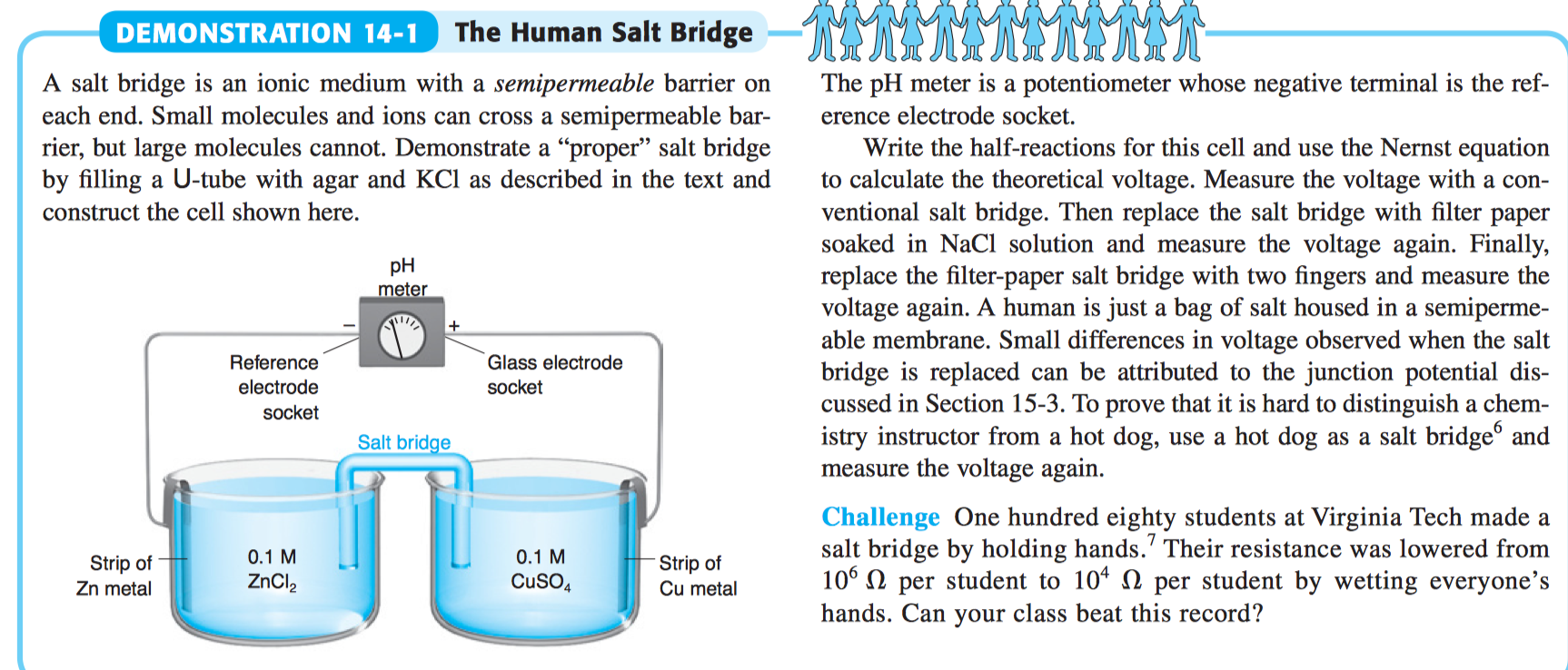
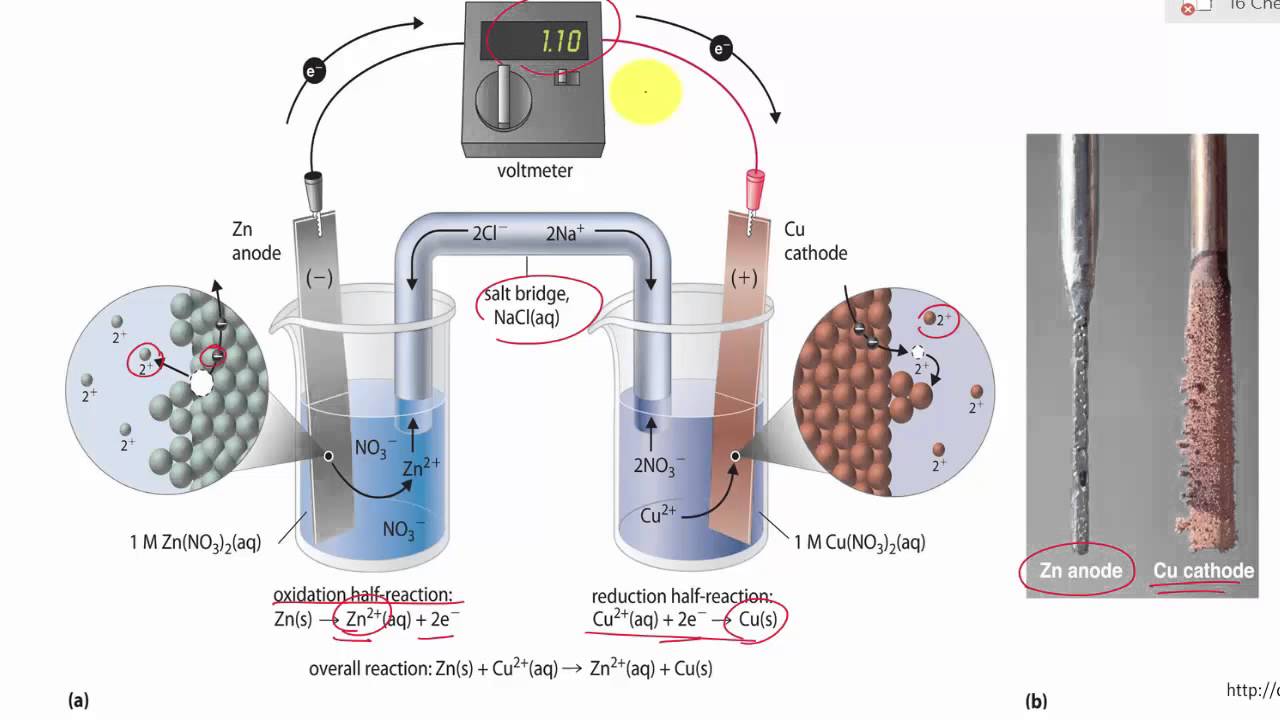
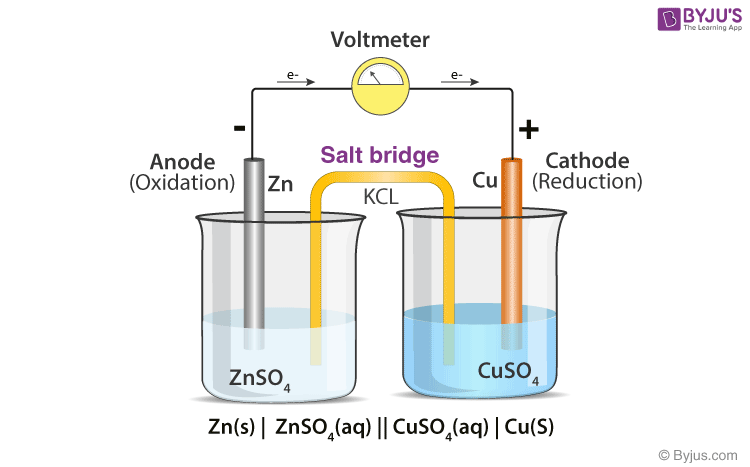
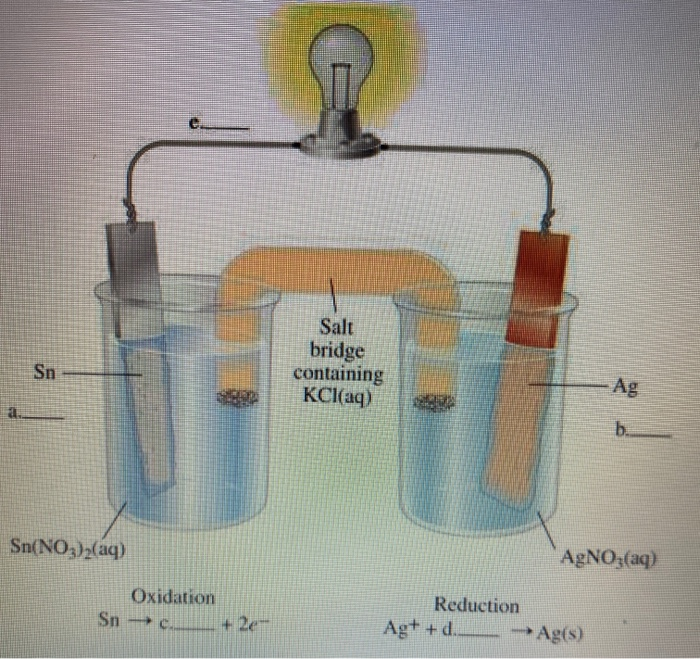
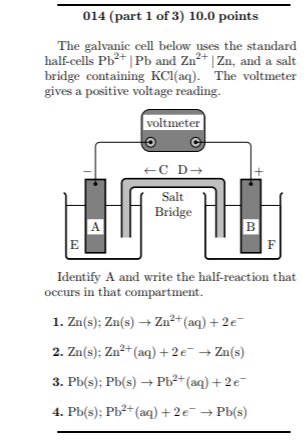
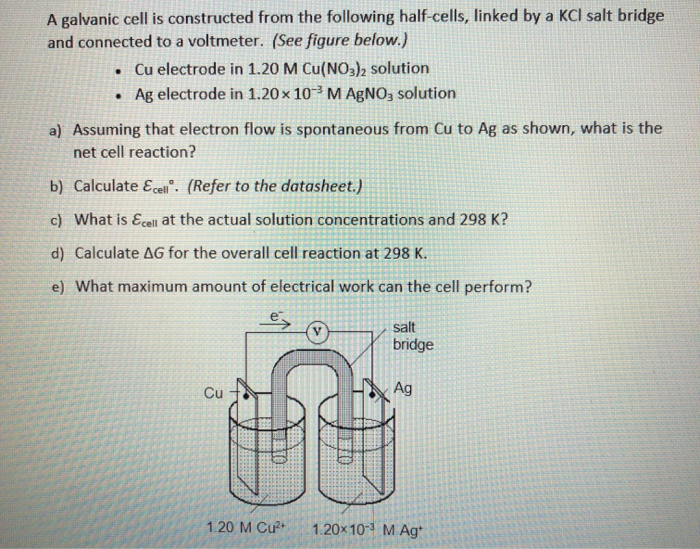
A salt bridge is used in voltaic cells to balance the ions and complete the circuit. Describe this scenario. (hint;use a diagram) | Homework.Study.com

Statement I: KCl, NaCl, NH4Cl, etc., cannot be used in the salt bridge of a cell containing silver.Statement II : A salt bridge contains concentrated solution of an inert electrolyte like KCl,

A) Schematic of the micro-agar salt bridge. Three percent agarose in 3... | Download Scientific Diagram

A) Schematic of the micro-agar salt bridge. Three percent agarose in 3... | Download Scientific Diagram

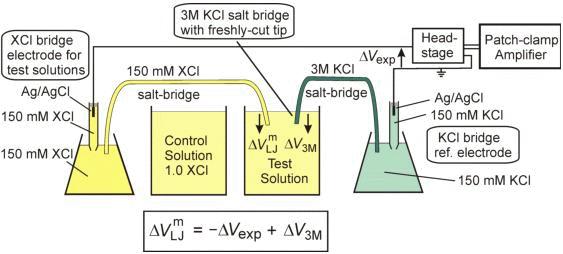
Reference Electrodes with Salt Bridges Contained in Nanoporous Glass: An Underappreciated Source of Error | Analytical Chemistry
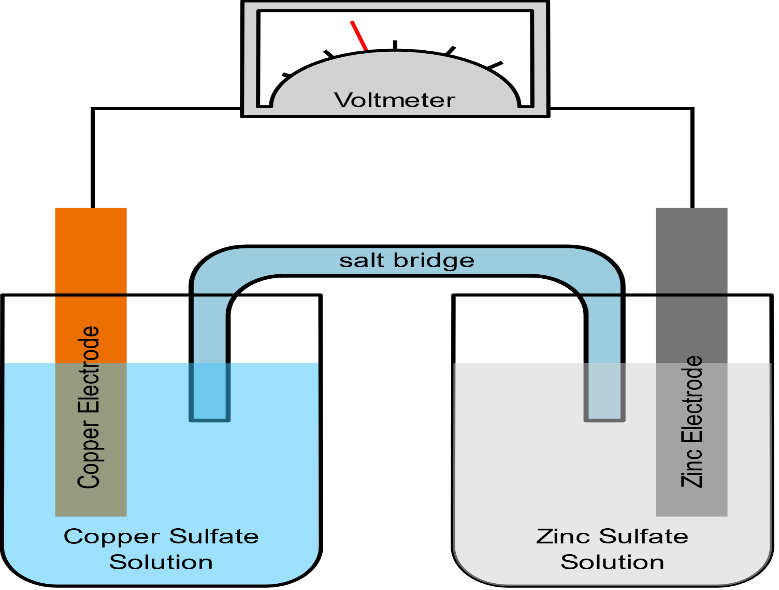
The function(s) of salt bridge in a cell is\/areA. It maintains standard electrode potential of cell constant which depends on several factors.B. It completes the electrical circuit.C. It departs both the solutions