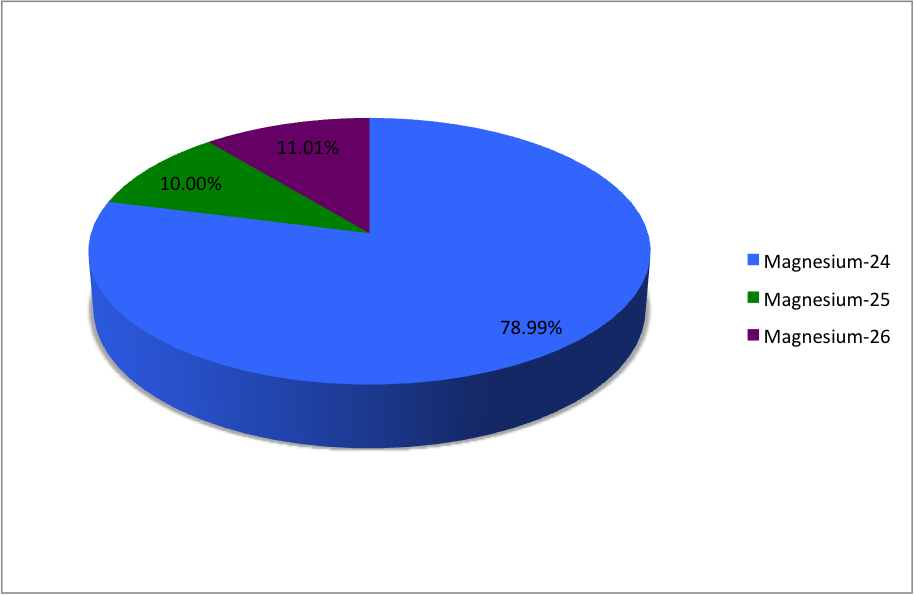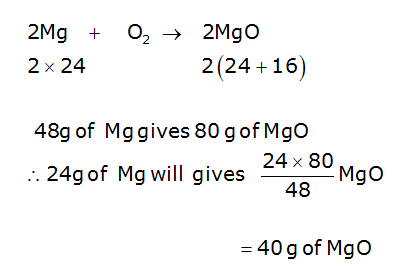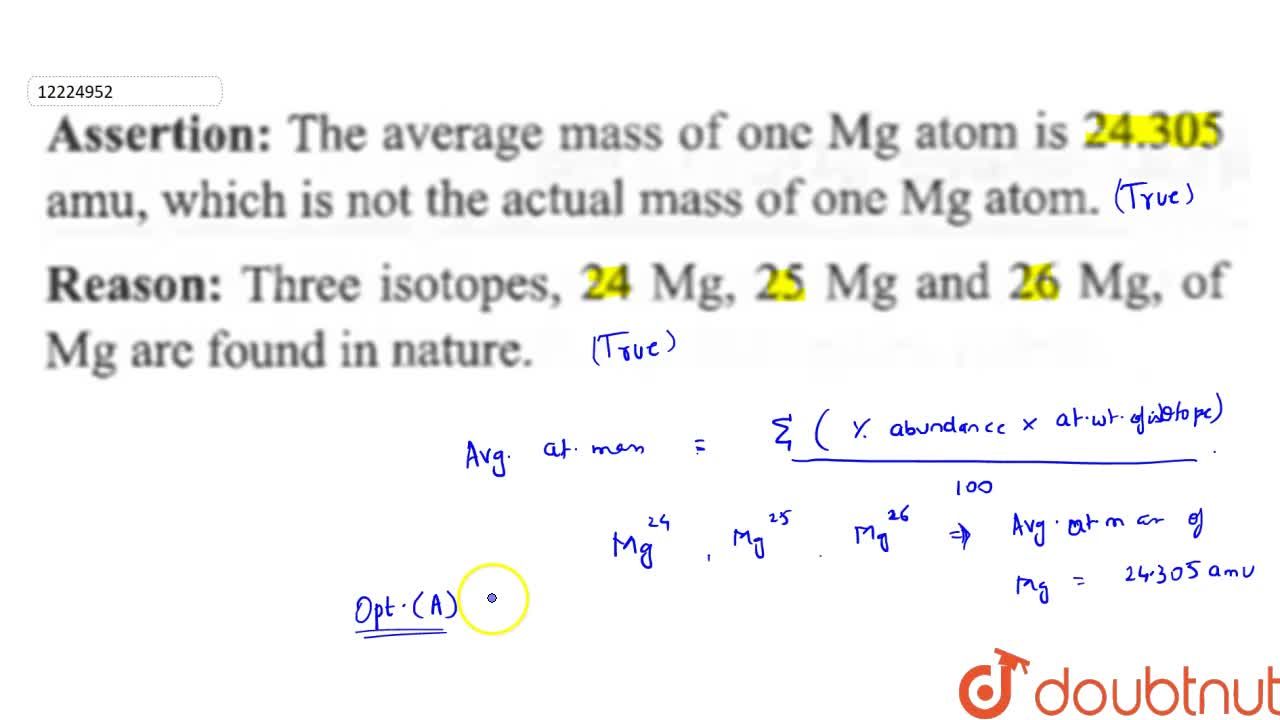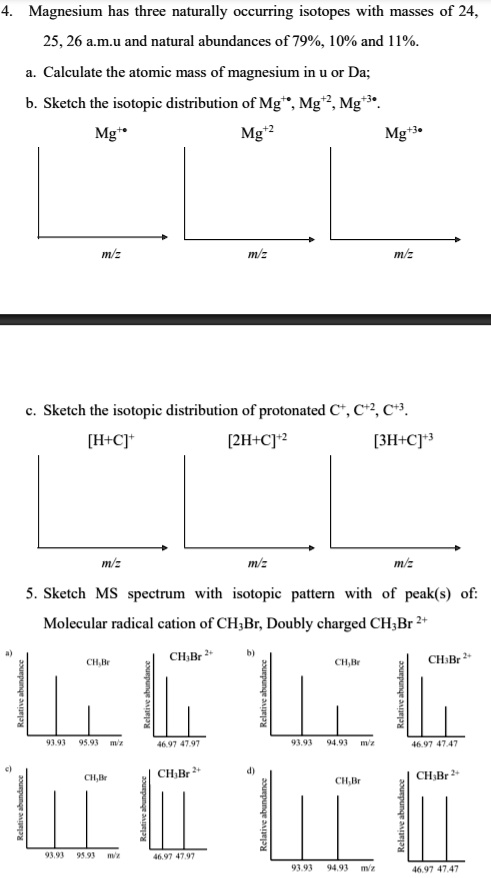
SOLVED: Magnesium has three naturally occurring isotopes with masses of 24, 25,26 a.m.u and natural abundances of 79%, 10% and 11%. Calculate the atomic mass of magnesium in U or Da; Sketch

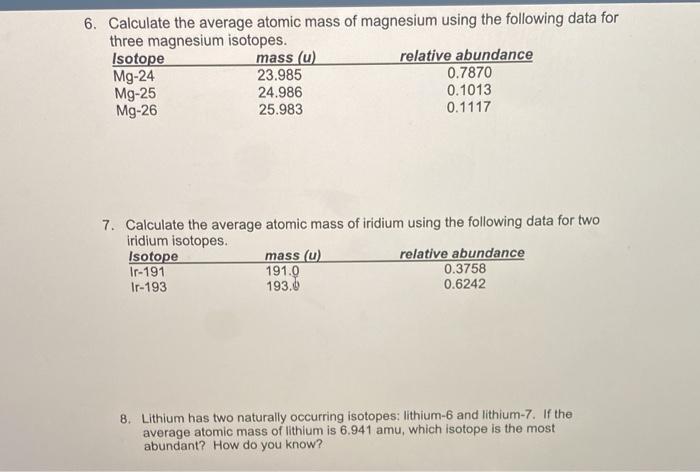
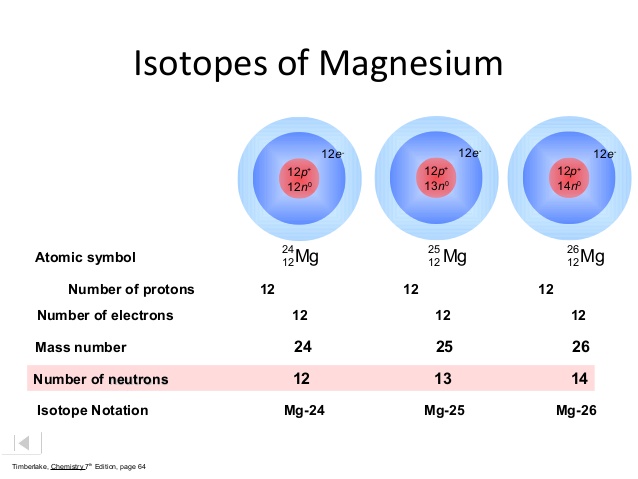
1 Warm Up Isotopes Mass of Isotope Abundance 24 Mg =24.0 amu 78.70% 25 Mg = 25.0 amu 10.13% 26 Mg = 26.0 amu 11.17% Calculate the mass average of magnesium. - ppt download

Magnesium and oxygen combine in the ratio of 3:2 by mass to form magnesium oxide. What mass of oxygen gas would be required to react completely with 24 g of magnesium?

In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their

Average atomic mass of magnesium is `24.31`amu. This magnesium is composed of 79 mole % of `24mg... - YouTube

Average atomic mass of magnesium is 24.31 amu. This magnesium is composed of 79 mole % of .^(24)Mg and remaining 21 mol % of .^(25)Mg and .^(26)Mg. Calculate mole % of .^(26)Mg.