The following reaction takes place when aluminium powder is heated with MnO(2) 3MnO(2)(s)+4Al(s)to3Mn(l)+2Al(2)O(3)(l)+"Heat" (a) Is aluminium gettuing reduced ? (b) zIs MnO(2) getting oxidised ?

Commercial aluminum powders, Part I: Particle size characterization and slow heating rate thermal analysis - ScienceDirect

Minerals | Free Full-Text | Hydrometallurgical Production of Electrolytic Manganese Dioxide (EMD) from Furnace Fines

What happens when (write reaction only)i) Steam is passed over hot aluminium.ii) iron (III) oxide (Fe2O3) heated with a reactive metal Aluminium .
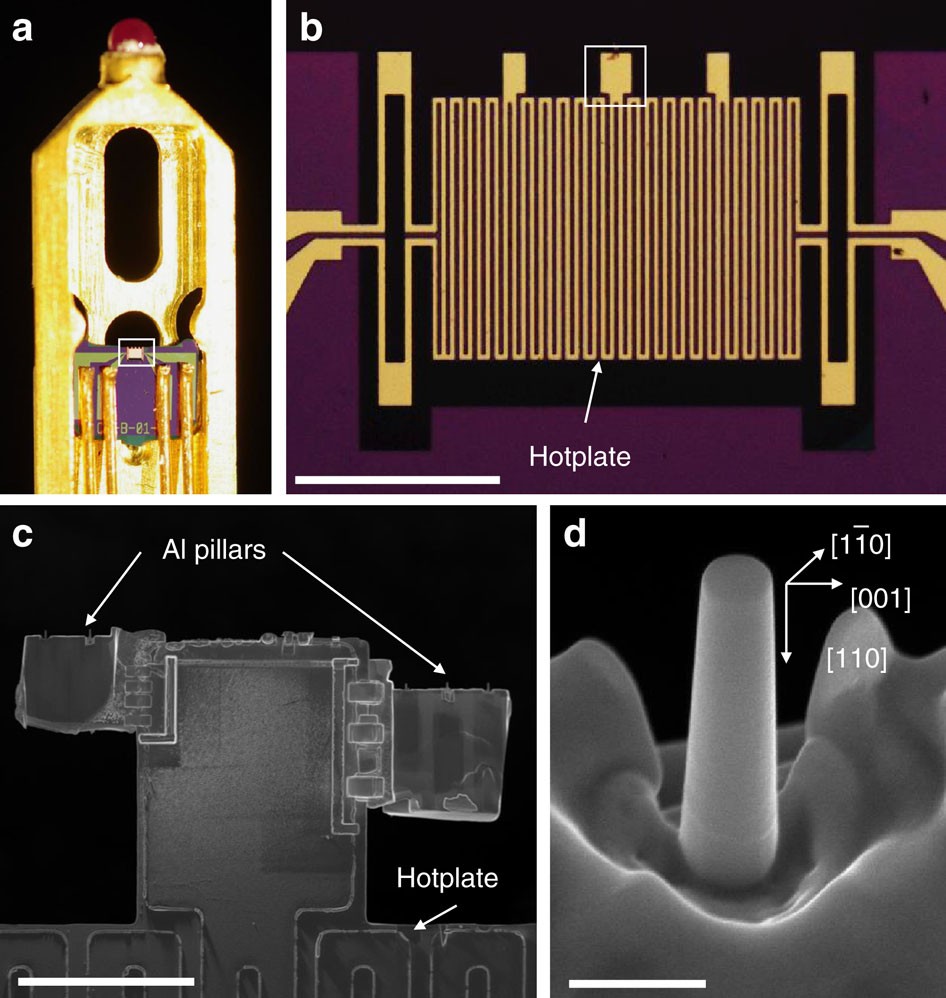
Effect of hydrogen on the integrity of aluminium–oxide interface at elevated temperatures | Nature Communications

The following reaction takes place when aluminium powder is heated with MnO(2) 3MnO(2)(s)+4Al(s)to3Mn(l)+2Al(2)O(3)(l)+"Heat" (a) Is aluminium gettuing reduced ? (b) zIs MnO(2) getting oxidised ?

Had a go at making thermite. It was very difficult to ignite with magnesium. Any advice to improve the process? : r/chemistry

a) Write the chemical reactions taking place when : (i) Manganese dioxide is heated with aluminium powder (ii) Steam is passed over red hot iron (c ) Magnesium reacts with hot water. (

ZnS → solubility in water = 0.97g K we mixed 2 moles of Zn(NO3)2 is 5 L solution. Find new solubility of Zn?





.jpg)