Why Magnesium has least melting amd boiling point among group 2 elements? And why calcium has least density?
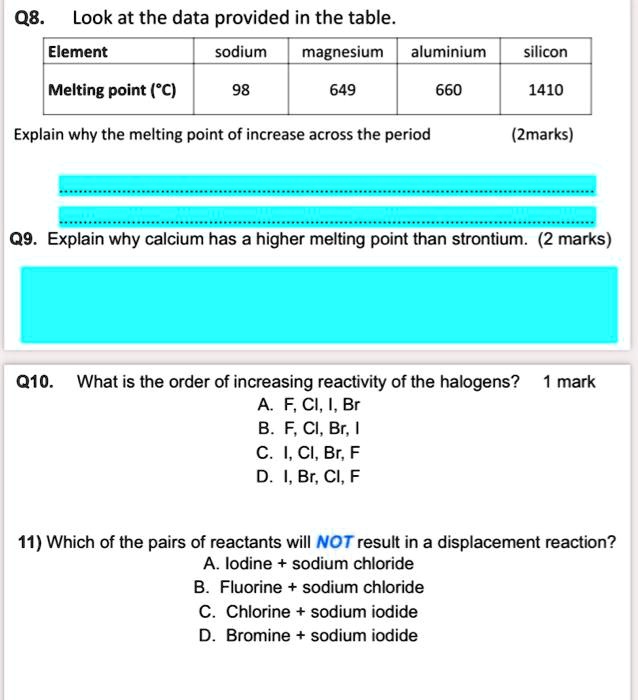
SOLVED: Look at the data provided in the table: Element sodium, magnesium, aluminium, silicon Melting point (°C): 98, 649, 660, 1410 Explain why the melting point increases across the period. (Z marks)

Magnesium. Alkaline earth metals. Chemical Element of Mendeleev's Periodic Table. in square cube creative concept Stock Photo - Alamy

Question Video: Understanding the Difference in Boiling and Freezing Points between Magnesium Chloride and Sodium Chloride Solutions of the Same Concentration | Nagwa

The melting points of the Period 3 metals sodium and magnesium are shown below. What is the differences in the melting points of sodium and magnesium, using the model of metallic bonding?
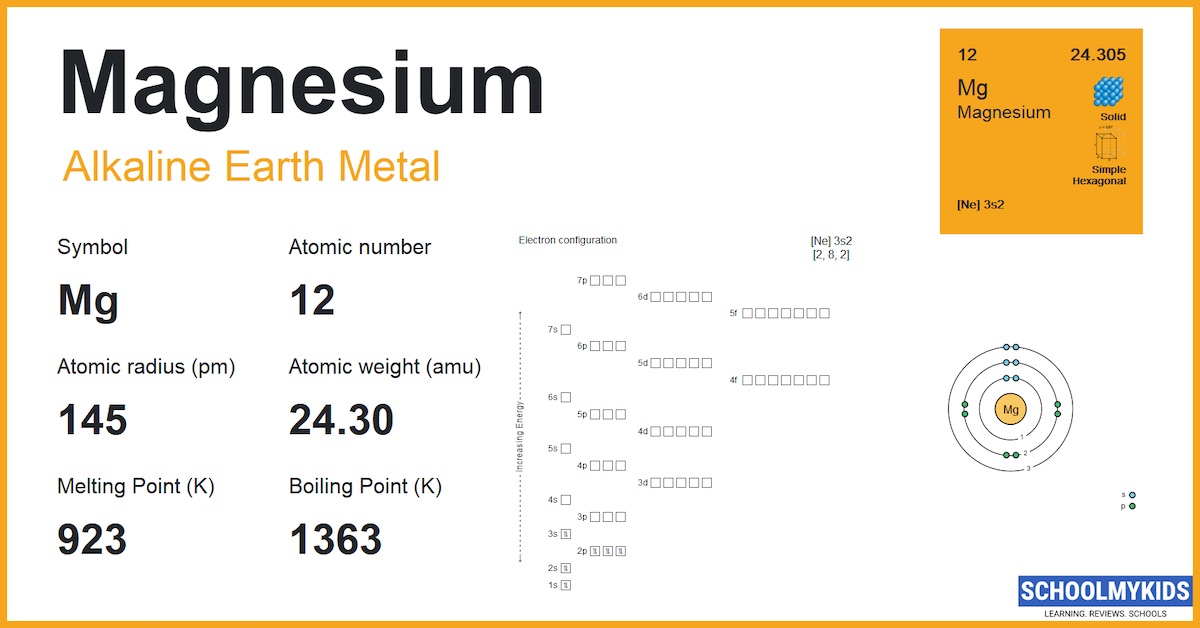
Mg Magnesium Element Information: Facts, Properties, Trends, Uses and comparison - Periodic Table of the Elements | SchoolMyKids

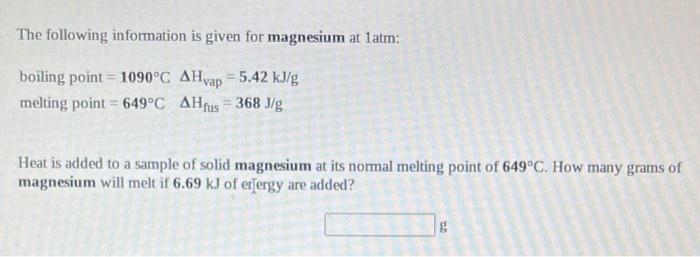
SOLVED: The following information is given for magnesium at 1 atm: boling point=1090C Hyap1090C=5424J/g melting point=649.0C H649.0C=368.3J/g specific heat solid=1.017 g.C specific heat liquid=1.339 J g.c A 32.00 g Sample of liquid




:max_bytes(150000):strip_icc()/GettyImages-1135707671-640473b29d534e15a24491c0d6b2789e.jpg)





![Properties of pure and alloyed magnesium at its melting point [94]. | Download Table Properties of pure and alloyed magnesium at its melting point [94]. | Download Table](https://www.researchgate.net/publication/311957511/figure/tbl3/AS:614062450814976@1523415305341/Properties-of-pure-and-alloyed-magnesium-at-its-melting-point-94.png)

![Properties of pure and alloyed magnesium at its melting point [94]. | Download Table Properties of pure and alloyed magnesium at its melting point [94]. | Download Table](https://www.researchgate.net/profile/Vyasaraj-Manakari/publication/311957511/figure/tbl3/AS:614062450814976@1523415305341/Properties-of-pure-and-alloyed-magnesium-at-its-melting-point-94_Q320.jpg)