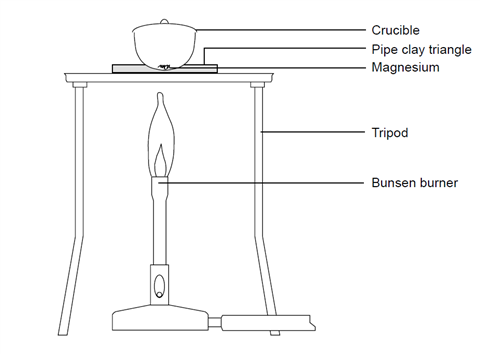
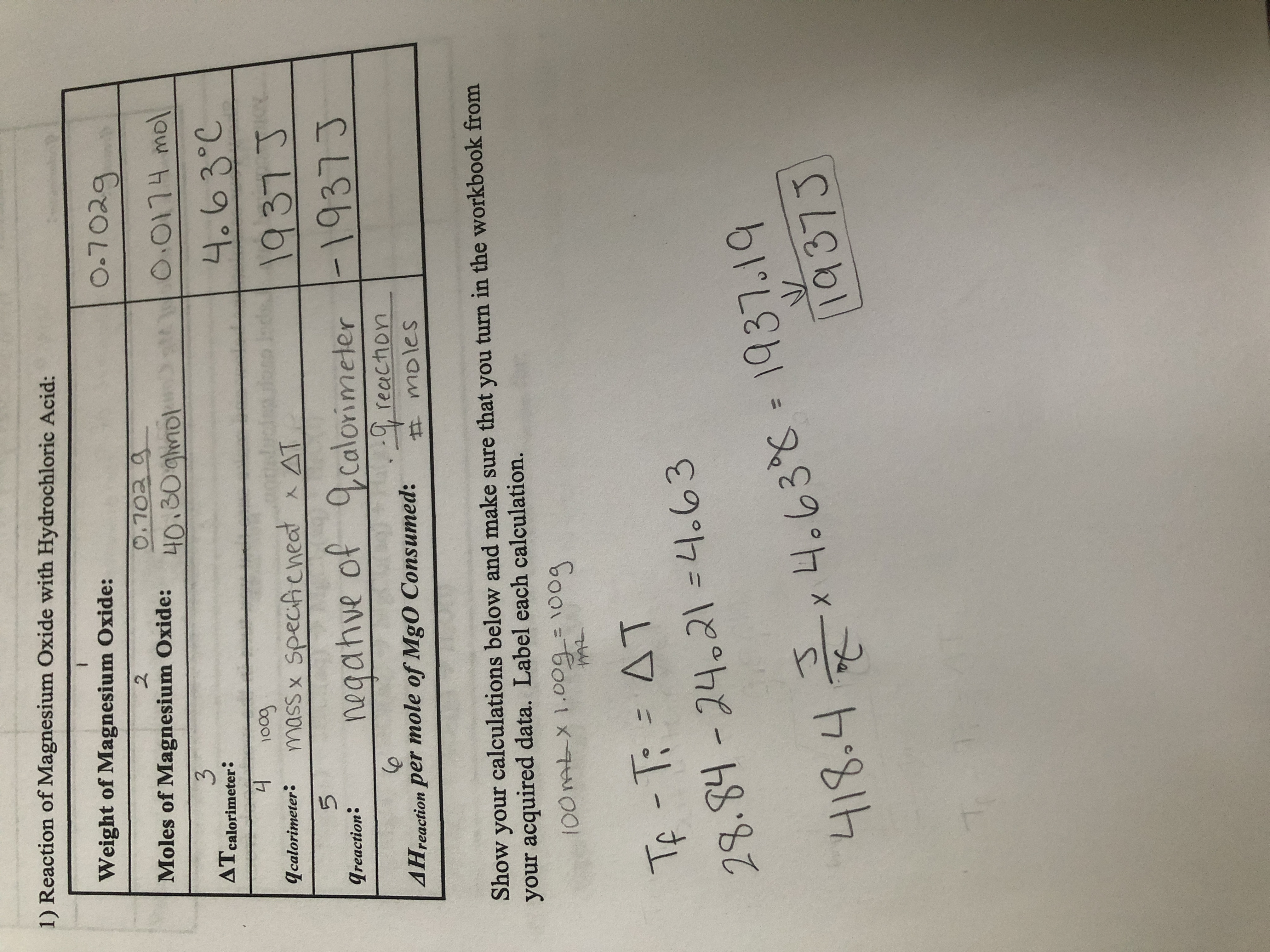
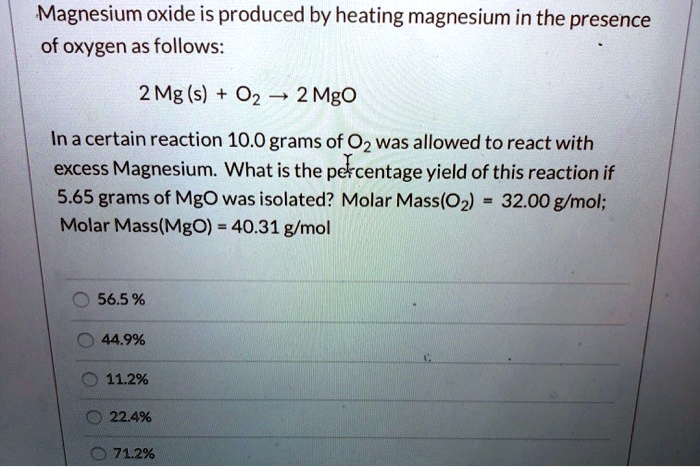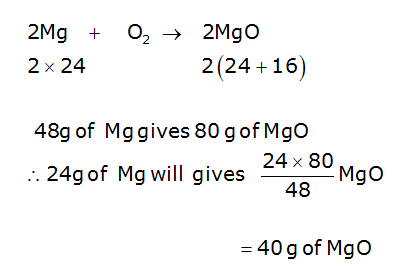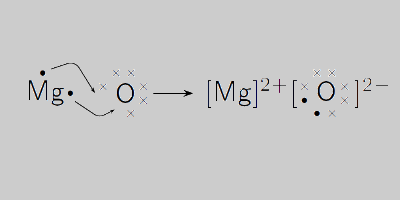Question Video: Calculating the Mass of Oxygen Required to React with a Given Number of Magnesium Atoms | Nagwa

OB: Intro to the Mole You gotta have a reference table and a calculator of your own now. No exceptions. - ppt download

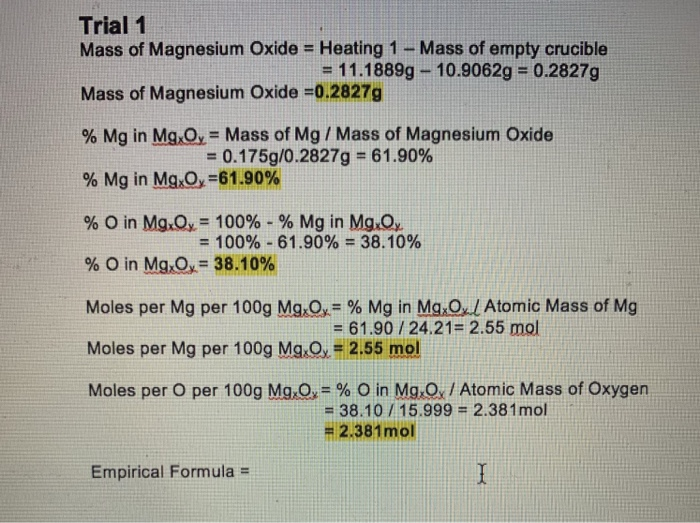
DOC) IB Chemistry IA: Determining the Empirical Formula of Magnesium Oxide | Josephine Yeh - Academia.edu
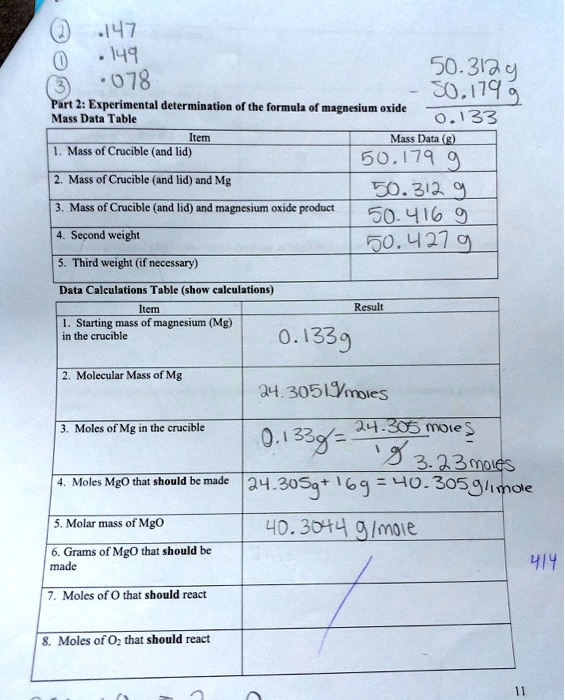
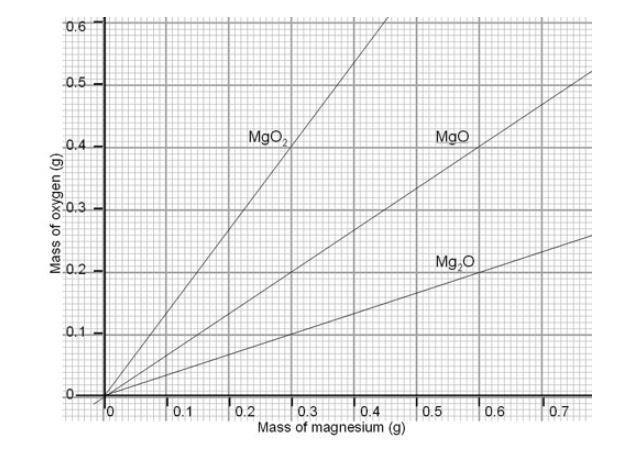
SOLVED: .147 149 50.313,9 078 50.179 Part 2: Experimental determination of the formulz of magnesium oxide Mass Data Table 0.133 Ms? Data Mass of Cricible (and lid) 50.179 Mass of Crucible (and
A 3.250g sample of magnesium is burned in a container of 12.500g oxygen. What mass of oxygen gas remains unreacted after the magnesium has been completely consumed to form magnesium oxide as

1a.calculate the relative formula mass of magnesium oxide when the relative atomic masses are O=16 - Brainly.com
![Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ] Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ]](https://toppr-doubts-media.s3.amazonaws.com/images/9085118/105391b5-2617-461a-9970-1a42367b289a.jpg)
Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ]
![Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ] Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ]](https://dwes9vv9u0550.cloudfront.net/images/8090905/68318e51-f37d-486f-b3fb-f22fbc1043ce.jpg)
Calculate the number of molecules present in 0.5 moles of magnesium oxide ( MgO) . [Atomic weights : Mg = 24, O = 16 ]